Abstract
Introduction
Lymphoblastic lymphoma (LBL) is a rare and aggressive form of Non-Hodgkin's Lymphoma (NHL) representing only 1-2% of overall cases. LBL consists of B-cell (B-LBL) and T-cell (T-LBL) variants with T-LBL accounting for 90% of adult cases. LBL is felt to represent the nodal disease equivalent to acute lymphoblastic leukemia (ALL). In fact, the WHO Classification of Tumors of Hematopoietic and Lymphoma Tissues classifies LBL and ALL as a single entity with variants based on genetic and molecular phenotype. As with many other centers, the Princess Margaret Cancer Centre has employed a similar treatment strategy for LBL as with ALL given their biological similarities.
Methods
We conducted a retrospective analysis of 43 patients at Princess Margaret Cancer Centre with LBL who initiated treatment between 1995 and 2015. Patients had a histologically confirmed diagnosis of Pre-T Lymphoblastic Lymphoma, Pre-B Lymphoblastic Lymphoma or Lymphoblastic Lymphoma NOS confirmed by a hematopathologist at the University Health Network. Patients were treated with a variety of regimens including dose intensive or paediatric ALL protocols and less dose-intensive (NHL) protocols. The primary endpoint was the rate of overall survival (OS) at 5 years. Survival times were calculated from chemotherapy start date to last follow-up date. Plots were created using Kaplan-Meier and Log Rank test was used to compare patient groups' survival experience. Secondary endpoints included the rates of complete remission (CR), progression-free survival (PFS) at 5 years and the frequency of treatment-related toxicities and their impact on OS and PFS. We hypothesized that age was likely an important factor in determining toxicity as well as treatment efficacy.
Results
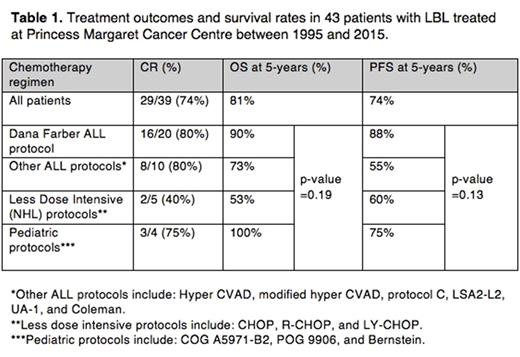
A total of 43 patients with LBL were identified in our database. The median age was 33 with a range of 5-83 years and the male-to-female ratio was approximately 3:1. The majority of patients (88%) had T-LBL subtype and 24 patients (56%) were stage IV at presentation. With respect to chemotherapy regimen, 23 patients (53%) received Dana Farber, 11 patients (26%) received another ALL-type regimen, a pediatric protocol was given in 4 patients (9%) and 5 patients (12%) received a less-dose intensive (NHL) regimen.
A complete response (CR) was seen in 29/39 (74%) of patients and a partial response (PR) seen in 7/39 (18%) of patients. Rates of overall survival (OS) and progression-free survival (PFS) at 5 years were non-significantly higher in patients treated with ALL protocols including the Dana Farber (DF) protocol (90% and 88% respectively) compared to less dose-intensive regimens (53% and 60% respectively). A statistically significant difference in OS and PFS in patients less than or equal to age 50 was seen compared to those greater than age 50. On the other hand, clinical stage, LDH level at diagnosis and ECOG performance status did not have prognostic significance.
With respect to toxicity, 14 patients experienced a total of 24 toxicity events of interest including venous thromboembolism, hypersensitivity reactions, febrile neutropenia, ileus and other gastrointestinal complications. In those who received DF, 10/23 (43%) of patients experienced a toxicity compared to 4/20 (20%) of patients who received a regimen other than DF (p-value=0.12). Of note, toxicity had no impact on 5-year OS as 85% patients with a toxicity outcome survived at 5 years compared to 80% without a toxicity outcome (p-value=0.49).
Conclusion
ALL treatment protocols have higher rates of OS and PFS in patients with LBL compared to less dose-intensive protocols with no significant difference in toxicity. Given the balance of benefit (favorable OS, PFS and CR rate) with aggressive ALL protocols versus the risks of toxicity (higher rates which do not appear to negatively influence OS), we believe our data favors treatment with ALL regimens in younger adults with LBL. Future research on LBL should assess the treatment experience at other centers given the relative rarity of this disease as well as assess long-term efficacy and toxicity outcomes.
Kukreti: Celgene: Honoraria; Amgen: Honoraria. Crump: Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Ortho: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kuruvilla: Roche: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Seattle Genetics: Consultancy, Honoraria; Hoffman LaRoche: Consultancy; Janssen: Consultancy; Gilead: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding; Roche: Research Funding; Celgene: Honoraria, Research Funding; Lundbeck: Honoraria; Merck: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal